| | Background | Objectives | Methods | Phosphorus terminology | Phosphorus forms referred to in this report | Assumptions used in this report
Background
A recent provincial water quality study showed that agriculture was adversely impacting water quality in Alberta (CAESA, 1998). The livestock industry is undergoing considerable expansion in Alberta. To maintain a marketing advantage by promoting high quality Alberta meat produced in a healthy environment, the livestock industry needs to be environmentally sustainable. As livestock production increases, the need to maintain public and consumer confidence that the industry is environmentally responsible becomes more acute. By 1999, both the industry and the public requested that a process be initiated to develop regulations for the industry.
Phosphorus (P) is a key element in environmental sustainability. While P is an essential element for crop nutrition, it is also the limiting nutrient in accelerated eutrophication of water bodies. The potential for soil P loading to degrade water quality was reviewed by Howard et al. (1999). According to Sims (2000), crop production increases with soil P levels to an optimum, beyond which there is no appreciable gain in yield; however, once soil P levels exceed the optimum for crop production, the risk to water quality increases (Figure 1.1). A major difficulty in managing for both economic and environmental sustainability is to know the levels of soil P that are optimum for crop growth and the levels that present an unacceptable risk to water quality.
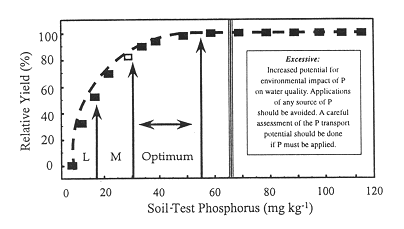
Figure 1: Generalized representation of the relationship between soil test P, crop response and the potential for an impact on water quality (Sims 2000).
Livestock manure is a major source of P. If manure is applied on land, it can potentially reduce the amount of commercial fertilizer required for crop production. If commercial fertilizer or manure is applied in strict accordance with agronomic recommendations based on soil test P values and crop demand, soil P levels are expected to increase minimally and the risk to water quality will likely remain acceptable (Sims 2000). However, in many cases there is insufficient land within an economically feasible distance to apply manure at rates that would meet but not exceed recommended agronomic rates for P. Acquiring additional land or transporting manure to more distant land can be costly. Thus, in the past, manure was applied in excess of crop P requirements on some fields. As a result, P has accumulated in these soils.
The Livestock Regulatory Stakeholder Advisory Committee requested that a study be undertaken to identify environmentally safe P loading limits for soils in the agricultural area of Alberta. These limits could be used as guidelines for P application from all sources, including livestock manure, commercial fertilizer, food processing waste, and municipal waste. The study is currently in progress and is being led by the Soil Phosphorus Limits Steering Committee, which is composed of industry and government members.
One of the guiding principles for the Steering Committee was to ensure that P loading rates would not impede optimal crop production. Several studies have reported that crops no longer respond to soil P levels beyond a threshold (Pierzynski and Logan 1993; Qian et al. 1994; Ashworth and Mrazek 1995; McKenzie et al. 1995; Johnston and Poulton 1997). It was expected that agronomic threshold levels would be less than environmental risk levels except in a limited number of very sensitive areas.
This review was carried out at the request of the Soil Phosphorus Limits Steering Committee to identify the agronomic threshold levels of soil P for Alberta so that these levels could be incorporated into the study to develop soil phosphorus limits for agricultural land in Alberta.
Objectives
The objectives of this report were to review:
- the basis for P fertilizer recommendations in Alberta ,
- the agronomic thresholds for P that apply to different crops and soil types in Alberta, and
- the implications for the application of organic P sources.
For the purpose of this review, an agronomic threshold for soil P is defined as the soil P level, determined by soil test P analysis, beyond which there is no practical economic or crop yield response to added P from either commercial (inorganic) fertilizer or organic fertilizer sources.
Methods
The information to address each objective of this report was initially obtained from an exhaustive review of the current literature. More than 200 sources were reviewed. Emphasis was placed on obtaining recent information from the Northern Great Plains of Canada and the United States.
The literature review on agronomic thresholds revealed two reports (McKenzie et al. 1995; McKenzie et al. 2001b) that interpreted wheat, barley, canola and pea yield response to soil P for Alberta. McKenzie et al. (2001b) provided their original raw data on pea yield response to soil P in Alberta.
This information was highly relevant but did not precisely define any agronomic thresholds; therefore, an analysis was developed to define agronomic thresholds from the data. The analysis calculated the yield increase for each 10 mg kg-1 increase in soil test P. A yield increase of 1% or less for a 10 mg kg-1 increase in soil test P was considered to be the zero return point because the yield increase was insignificant compared to the cost of raising the soil P level by 10 mg kg-1. This zero return point was used as the agronomic threshold which was then compared to agronomic thresholds reported in the literature.
Phosphorus terminology
Phosphorus concentrations can be expressed in several ways, and no standard for the terms of expression has been adopted. Phosphorus is usually expressed as concentration of elemental phosphorus in soil and water, and as P2O5 in commercial fertilizers. It can also be expressed as orthophosphate (PO4-3). Phosphorus concentrations in soil are often expressed as mg kg-1 of soil, which is the same as parts per million (ppm). Phosphorus content in water, because of the sensitivity of water to low concentrations, is often reported as mg L-1, which is the same as parts per billion (ppb). In this report, phosphorus in soil will be expressed as mg kg-1 of elemental P. The relationships between some of the units are presented in Table 1.1.
Table1 :Conversion factors for some common phosphorus reporting terms.
mg L-1
mg L-1
mg kg-1
ppb
P2O5
P | = ppb
= ppm
= ppm
= 1000 * ppm
= P * 2.2914
= P2O5 * 0.4364 |
Note: the equality between mg L-1 and ppm, and between mg L-1 and ppb assumes a solution in water where the specific gravity is near 1.0.
There are several methods for testing soil for plant-available P, each yielding a slightly different available P value. For consistency, all soil test P levels will be expressed in terms of the Norwest Labs’ Modified Kelowna method unless otherwise stated. Conversion formulas used in this report are based on relationships developed by McKenzie et al. (1995).
Phosphorus forms referred to in this report
Phosphorus exists in a variety of inorganic (Pi) and organic (Po) forms in the soil. Table 1.2 presents the abbreviations used to refer to P forms in this report. The same abbreviations are commonly used in the literature.
Table 2:Terminology and abbreviations used for the various forms of P.
Pt
Pi
Po
Pd
BAP
STP
PO4-3
Pp | = Total P
= Inorganic P
= Organic P
= Dissolved P
= Biologically available P
= Soil test P
= Orthophosphate
= Particulate P | = All forms of P
= Mineral forms of P
= Organic forms of P
= All forms of P dissolved in water
= P used by algal populations
= Correlated to plant-available P
= One form of solution P or Pd
= P sorbed to soil particles |
The forms vary in their solubility in water and availability to plants, and have been described in detail by Stewart and Sharpley (1987). The solution pool contains Pi and Po in forms used directly by crops and microorganisms. Labile P refers to readily available forms that can move into solution quickly as the P concentration in the solution pool is depleted by plants or microorganisms. Moderately labile forms include inorganic P that is associated with iron (Fe) or aluminum (Al), or moderately available organic forms, both of which are capable of contributing to the solution P pool. Slowly available forms include Pi associated with calcium (Ca) and magnesium (Mg), or primary mineral P. The least available forms include Po in particulate forms, which are occluded (unavailable), and Pi forms that are insoluble.
Chemical analyses to determine the proportion of the various forms of P in soils have been reported by Hedley et al. (1982), Tiessen and Moir (1993), and Qian and Schoenau (2000a). All analysis methods generally use an extractant to change the targeted P form(s) to orthophosphate PO4-3. The concentration of the orthophosphate can then be measured and related to the amount of the targeted P form in the soil. Since the forms of P are often referred to by the type of extraction procedure used to detect them, Table 1.3 presents the extractant used to isolate the particular form.
Table 3: Extractants for the various forms of P based on Qian and Schoenau (2000a).
| P form | Extractant |
| Soluble P | Distilled water |
| Labile (readily available) Pi | Resin |
| Labile (readily available) Pi and Po | NaHCO3 |
| Moderately labile P | NaOH |
| Slowly available P | 1 M HCl |
| Occluded Po (unavailable) | Conc. HCl |
| Residual (resistant) P | H2SO4 and H2O2 |
Assumptions used in this report
Nutrient concentrations in soil are often expressed in terms of kg ha-1 for a given depth within the soil profile. To allow comparisons, these units were converted from kg ha-1 to mg kg-1 in this report. This conversion requires that the depth and bulk density of the soil of interest be known or have assumed values. For the conversions in this report, soil depth was assumed to be 0.15 m, a common soil depth for nutrient analysis, and soil bulk density was assumed to be 1.2 Mg m-3.
For example, converting 100 mg kg-1 of P to a nutrient content in the top 0.15 m of the soil would require the following steps:
- Soil bulk density is assumed to be 1.2 Mg m-3
- 100 ppm = 100 mg kg-1
- (100 mg kg-1 * 10-6 kg mg-1 * 1.2 Mg m-3 * 10-3 kg g-1 * 106 cm3 m-3 * 104 m2 ha-1 * 0.15 m depth) = 180 kg ha-1
|
|