| | Soil tests for plant-available P | Determination of phosphorus response curves| Interpretations of crop responses to soil P and fertilizer P | Interaction and application factors | Residual P effects | Starter P fertilizer | Relationship between plant-available P and water quality risk
Soil test P results ultimately form the basis for crop response and fertilizer recommendations. Agricultural soil analysis techniques have been primarily used to identify total P (Pt ) or plant-available P, which is correlated with soil test P (STP). Crop fertility status and fertilizer requirements are based on STP; therefore, the test is widely used in the industry for routine soil analysis. Organic P (Po) forms are important when assessing soil P transformations, which can be applied to fertility, especially when considering manure. Other P fractions, including biologically available P (BAP), dissolved P (Pd) and particulate P (Pp), have become important factors when assessing the environmental impacts of soil P contributions; however, they have not generally been part of routine soil analysis.
Soil test P methods are based on using chemicals to dissolve enough P from the soil to estimate the amount of P the soil is capable of supplying to the crop. The main purpose of a soil P test is to determine an index of the amount of supplemental P required to prevent loss of crop value because of P deficiency (Fixen and Grove 1990). Plant uptake results in a decrease in solution P, which is replenished by dissolution of mineral P or release of adsorbed P. Soil conditions, especially pH and P sorption capacity, influence this process. The STP analysis uses chemical extractants to create solutions that dissolve P in amounts consistent enough to be correlated with crop P use. The amount of dissolved P can change for different soils and different pH levels.
Soil tests for plant-available P
Analysis of soil to detect P levels follows the general method of dissolving some or all of the soil P in an extractant, then determining the concentration of P in the extractant. A typical extraction procedure involves the shaking of a known weight of soil (e.g. 5 g) with a known volume of extractant (e.g. 50 mL) for a fixed period of time (e.g. 30 min.) at room temperature. The mixture is then filtered, and the filtrate is analyzed for extractable P (PO4-3) content. Measurement of plant-available P in filtered extractant is determined using an inductively coupled plasma (ICP) spectrophotometer, or colorimetrically using phosphomolybdic blue complex reduced with ascorbic acid (Watanabe and Olsen 1965). The ICP probe will respond to other forms of P as well as PO4-3, and can give some slightly higher results for some extracts (Miller and Kotubny-Amacher, in press).
The purpose of the test and the type of soils being tested determine the type of extraction and the method of soil sampling. For STP, soil tests are based on a cored or augered sample to 0.15 m depth. The results of the analysis are then correlated to crop growth or nutrient uptake. In cases where a particular soil P test does not correlate to crop response, either by research or field experience, it can eventually be replaced by a test in which the users have more confidence. For example, the Miller-Axley test, which has been used in the Alberta provincial laboratory for several years, has recently been reported as underestimating soil P availability (McKenzie et al. 1998). Most STP analyses in Alberta are now performed using modifications of the Kelowna test.
Bray and Kurtz (1945) and Olsen et al. (1954) used weak extractants, which correlated well with plant uptake. The Bray and Kurtz (1945) extraction method, referred to as Bray-1, initially used 0.03N NH4F and 0.025N HCl for determining "adsorbed-P". Fluoride was included in the extractant because it had a high capability to displace adsorbed P. Surface adsorbed P has a greater influence on P availability because its surface area is exposed to the chemical reactions, therefore, it more readily replaces the P in soil solution as solution P is removed by plant uptake (Thomas and Peaslee 1973). Since adsorbed and acid-soluble forms of P co-exist in soils, Bray and Kurtz (1945) proposed a stronger extractant using 0.1 N HCl instead of 0.025N HCl for determining both forms of P.
Relationships between crop response and the Bray-extractable P were not consistent across several soil types. Free carbonates in soils neutralize the acid in the extractant resulting in precipitation of dissolved P (Syers et al. 1987). Soils at pH values greater than 7.2 frequently contain enough free carbonates to at least partially neutralize the HCl in the Bray extractant (van Lierop 1988). Numerous extraction methods have been developed, all with advantages and limitations depending upon the soil type for which they have targeted. Their suitability relies on long-term correlation studies that have established relationships between the extraction method, the soil and crop response. Therefore some extraction methods are favored more in some parts of the world than in others. Soil pH, mineralogy and organic matter content are major factors in accuracy. The tests are differentiated by the extractant used. A summary of the more common tests is presented in Table 2.1.
In Alberta, the Miller-Axley and the Bray were the main extractions for available P, but both were unsuitable for the highly calcareous, high pH soils of southern Alberta because the free carbonates neutralized the weak acid in the extractant (McKenzie and Kryzanowski 1990). During the 1970s and 1980s, a second extraction using the Olsen (NaHCO3) test was performed in Alberta and British Columbia provincial soil laboratories to determine the available P in high pH soils (van Lierop 1988; McKenzie and Kryzanowski 1990). The Miller-Axley test was developed in the 1970s, before large amounts of commercial fertilizer P were added to soils. At that time, the chief sources of P were from soil minerals and organic sources (McKenzie et al. 1998).
The Kelowna extraction was proposed by van Lierop (1988) as a single test that would apply to a wide range of soil conditions. It could replace the Bray and Olsen tests. The extractant used acetic acid in place of the weak HCl used in the Bray and H2SO4 used in the Miller-Axley extractants. While the Kelowna extraction performs similar to the Olsen test at high soil pH levels, it does not require charcoal filtration and does not evolve CO2 (Qian et al. 1994).
In the Canadian prairies, modifications of the Kelowna test were tested against crop response in greenhouse trials (Ashworth and Mrazek 1989; McKenzie et al. 1989; Qian et al. 1994) and field trials (Ashworth and Mrazek 1989, 1995). The testing validated the Kelowna and modifications of the Kelowna for a variety of soil types. Addition of ammonium acetate to the Modified Kelowna extractant resulted in an improved prediction of available potassium (K) compared to the Kelowna (Qian et al. 1994). The Kelowna and its modifications are considered accurate for Canadian prairie soils (Havlin et al. 1999).
Table 1 : Summary of P analysis methods (modified from Sibbesen and Sharpley 1997, and Howard et al. 1999).
Analysis method | Extractant | Comments |
| Olsen | 0.5M NaHCO3 @ pH 8.5 | -best suited for neutral and calcareous soils (Qian et al. 1994)
-process of maintaining pH level, driving off CO2, and filtering extractant through activated charcoal makes the procedure awkward (Qian et al. 1994) |
| Mehlich-3 | 0.2M CH3COOH
0.25M NH4NO3
0.015M NH4F
0.013M HNO3
0.001M EDTAz | -common method for assessing crop- available P in U.S. |
| Bray-1 | 0.03N NH4F
0.025N HCl @ pH 3.5 | -designed for neutral - acidic soils
-not suited for calcareous soils |
| Bray (strong) | 0.03N NH4F
0.1N HCl @ pH 1.0 |  |
| Miller-Axley | 0.03N NH4F
0.03N H2SO4 | -not suited for calcareous soils |
| Modified Kelownay | 0.015M NH4F
0.25M ammonium acetate
0.25M acetic acid | -best method for a wide range of soil pH levels in the prairie provinces
-measures available P and K |
| Modified Kelownax | 0.015M NH4F
1.0M ammonium acetate
0.5M acetic acid | -best method for a wide range of soil pH levels in the prairie provinces
-measures available P and K |
| Kelowna | 0.015M NH4F
0.25M acetic acid | -suitable for a wide range of soil pH levels |
z EDTA is ethylene diamine tetraacetic acid
y Used by Enviro-Test Labs
x Used by Norwest Labs
Two modified Kelowna methods have become the most accepted and widely used tests in Alberta. While they have been correlated with each other in tests (Table 2.2) (McKenzie et al. 1995), there is no provincial standard. One method is used by Norwest Labs, and the other is used by Enviro-Test Labs. Ashworth and Mrazek (1995) report that the concentration of acetic acid in Norwest Labs’ extractant (0.5 M) reduces the risk of calcareous soils neutralizing the extractant.
A sample of equivalent STP values from common tests, derived from the equations in Table 2.2, is presented in Table 2.3. Comparison of tests can result in varying relationships due to differences in sample preparation and analysis techniques. An example is the regression equations developed by Qian et al. (1994):
ETMK = 0.82 + 0.92*Olsen (1)
ETMK = -2.28 + 1.52*Kelowna (2)
Where:
ETMK = Enviro-Test Labs’ Modified Kelowna test,
Olsen = Olsen test
Kelowna = Kelowna test
Table 2: Relationships between common STP methods and the Norwest method (McKenzie et al. 1995). The Norwest method is represented by x in the regression equations.
| Extraction method (y) | Regression equation | r2 |
| Enviro-Test Modified Kelownaz | y = 4.038 + 0.941x | 0.88 |
| Miller-Axley | y = 3.258 + 0.717x | 0.57 |
| Olsen | y = -3.319 + 0.886x | 0.76 |
| Kelowna | y = 1.915 + 1.137x | 0.86 |
| Resin | y = -16.23 + 1.938x | 0.51 |
| Bray-1y | y = -4.08 + 1.103x |  |
| Mehlich-3x | y = 13.004 + 1.037x | 0.97 |
z Used by Enviro-Test Labs
y Calculated from van Lierop (1988) for soils with pH < 7.0; no r2 determined
x From M. Amrani (personal communication)
Equation (1) predicts Enviro-Test Modified Kelowna STP values from the Olsen test that are lower than those predicted with the equations in Table 2.2. For example, from Table 2.3 a Norwest Modified Kelowna value of 10 mg kg-1 corresponds to an Enviro-Test Modified Kelowna value of 13 mg kg-1 and an Olson value of 6 mg kg-1. Equation (1) from Qian et al. (1994) predicts an Enviro-Test Modified Kelowna value of 10 mg kg-1 from an Olsen value of 10 mg kg-1.
Equation (2) predicts Enviro-Test Modified Kelowna STP values from Kelowna tests that are higher than those predicted with the equations in Table 2.2. From equation (2), a Kelowna value of 40 mg kg-1 will predict an Enviro-Test value of 58 mg kg-1. Using Table 2.3, a Kelowna value of 47 mg kg-1 corresponds to an Enviro-Test value of 42 mg kg-1. This suggests that the cross-referencing of STP methods requires careful design to ensure that variability between soils and lab techniques have been accounted for and standardized.
Table 3: Calculation of STP values from values of Norwest Labs’ Modified Kelowna analysis.
Norwest
STP | Enviro-Testz
STP | Miller-Axleyz
STP | Olsenz
STP | Kelownaz
STP | Bray-1y
STP |
10 | 13 | 10 | 6 | 13 | 7 |
20 | 23 | 18 | 14 | 25 | 18 |
40 | 42 | 32 | 32 | 47 | 40 |
60 | 60 | 46 | 50 | 70 | 62 |
80 | 79 | 61 | 68 | 93 | 84 |
100 | 98 | 75 | 85 | 116 | 107 |
z Based on equations from McKenzie et al. (1995).
y Based on equations modified from van Lierop (1988).
Ion exchange strips have been proposed by Qian et al. (1992) and Schoenau et al. (1993) as a technique for assessing available nitrogen (N), P, K, and sulfur (S). The strips adsorb ions directly from solution in a manner similar to uptake by roots. The strips are designed to be buried in situ and mimic root ion exchange.
A resin exchange probe, which uses the same principle, is now in commercial use by Western Ag Labs in Saskatoon, Saskatchewan, and has been tested in more than 600,000 ha of agricultural land in western Canada (J. Schoenau, personal communication). The probe offers the advantage that it measures P availability from the soil solution with time and in situ. Therefore, changes in P availability as a result of temperature changes and other site factors can be detected. Probes have had limited use in manured soils at this time; however, ease of use and potential for a closer approximation of crop nutrient uptake may result in this method becoming widespread for determination of STP.
Determination of phosphorus response curves
Crop yield response to STP levels increases to a critical level, beyond which yield does not increase appreciably regardless of how much more P is applied (Qian et al. 1994; Ashworth and Mrazek 1995; McKenzie et al. 1995; Johnston and Poulton 1997). In the field, crop response is determined by several factors including soil, nutrient status, cropping history, management and climate.
Kastens et al. (2000) modeled wheat response to STP and observed that STP had the most influence on wheat yield, even more than fertilizer P. Soil testing was used to obtain values for nutrients that would help predict the amount of nutrients needed to supplement the nutrient supply in the soil. Although a crop response to fertilizer will not always be obtained in a soil of low nutrient status, because of environmental and other limiting factors, a low nutrient status soil has a greater probability of response than a soil with a high nutrient status (Havlin et al. 1999).
Interpretations of crop responses to soil P and fertilizer P
Soil tests for fertility assessment are generally performed on soil sampled to 0.15 m depth from representative parts of a field. Interpretation of soil test results is used to predict the potential effect of soil nutrient levels on crop growth. STP levels have been linked to crop use of P by assessing crop response in plots and/or measuring P in plant tissue analysis. Crop response interpretations are generally based on controlled plot experiments. Crop characteristics in a control plot set, with no fertilizer added, are measured and compared with enough treatment sets, with incremental amounts of fertilizer added, to assess optimum crop response. Crop growth stage characteristics are recorded but the primary comparison is to yield data, with the degree of response determined by the treatment yield-control yield ratio. This method is used for assessing crop nutrient requirements for optimum yield, whereas tissue analysis can be used to quantify nutrient uptake, and identify deficiencies and processes. Nutrient uptake by the herbage of pasture and hay crops has been considered a good method for estimating the quantity of fertilizer required for the crop in the next year (Nuttall 1980; McCartney et al. 1998).
The degree of sophistication for interpreting soil test results increases with the effort put into researching and understanding the relationship between soil tests and crop growth. The Alberta Soil Test Advisory Group (1998) identified three levels of sophistication in soil test interpretation. Initially, when a soil test is developed it only indicates whether there is a deficiency (limitation) or a sufficiency (no limitation) of a nutrient. For that nutrient, this results in one of two recommendations, no fertilization or an amount considered adequate.
The second stage of development is to divide the soil test values into categories. Soil tests for available nutrients are often categorized as being low, medium, or high, based on the probability of response to application of the nutrient in question. An example is presented in Table 2.4.
Table 4: Example of the second stage of development of soil test interpretation where soil test results are split into three categories (Alberta Soil Test Advisory Group 1998).
Soil test level | Probability of response | Fertilizer required |
Low | High | High |
Medium | Medium | Medium |
High | Low | Low |
The final stage is to fully calibrate the soil test values with crop response using regression techniques so that an infinite number of categories could be obtained (Barber 1973). For calibration, the relationship of the soil test result for an available nutrient to crop growth is based on how much of that nutrient is utilized by the crop. The soil test result provides only a relative measure of nutrient supply as correlated to crop response (Alberta Soil Test Advisory Group 1998) and should be considered an index of nutrient availability and potential yield response (Havlin et al. 1999).
Because of the many factors influencing crop growth, interpretation of crop response data for fertilizer recommendations must consider environmental factors such as soil moisture, precipitation, temperature, local soil and landscape conditions, crop rotation and cropping history (Ashworth and Mrazek 1995; McKenzie et al. 1995). Recommendations from some soil test labs include input from local sources, such as the producer or local fertilizer agent. Fertilizer recommendations should consider all these factors, plus economic conditions, and the goals of the producer. Barber (1973) identified four methods for determination of P fertilizer rates based on the objectives of the producer:
- adding enough P to build the soil to the desired level for the most economic production for several years (e.g. a rate of 60 kg ha-1 for a corn crop on a soil with low P levels).
- adding enough P to give the maximum net return on the immediate crop, neglecting residual benefits (e.g. a rate of 30 kg ha-1 for a corn crop on a soil with low P levels).
- fertilizing for a period of 3 to 4 years at a uniform annual rate that will give the highest net return for the period (e.g. an annual rate of 20 kg ha-1 for the period, for corn crop on a soil with low P levels).
- fertilizing for a period of 3 to 6 years by calculating the total requirement for the period and then applying the P fertilizer at the most effective times to realize the greatest net return for the period (e.g. a rate of 45 kg ha-1 initially for a corn crop on a soil with low P levels, followed by perhaps one other application during the period).
Therefore, fertilizer recommendations for the same soil test levels and crop can vary depending on local conditions, economics and the goals of the producer.
The philosophy in Alberta is to only recommend application of nutrients lacking in the soil (Alberta Soil Test Advisory Group 1998). Soil test recommendations are based on field research, which measured yield increase from several rates of fertilizer applied to soils with various soil test levels. To achieve optimum yields, large quantities of fertilizer need only be applied to soils with low test levels, moderate quantities of fertilizer to soils with medium test levels and low quantities to soils with high test levels. Recommendations are specific for the crop to be grown and the soil-climatic area. The recommendations are designed only for the next growing season, and this allows determination of economic returns based on current prices.
Interaction and application factors
Crop utilization of P is significantly enhanced when the appropriate balance of nutrients is available. Nitrogen, in particular, enhances P uptake by increasing top and root growth, altering the plant metabolism and increasing the solubility and availability of P (Tisdale et al. 1985). Crop uptake of P also increases with increasing soil temperature, moisture, aeration and soil biological activity. Phosphorus availability to crops is most favorable in a pH range of 6.0 to 6.5 (Havlin et al. 1999). Availability is decreased at low pH levels by oxides of iron and aluminum, and at high pH by calcium and magnesium.
Fertilizer application methods also influence crop response. McKenzie et al. (1998) examined P response of irrigated alfalfa from 1994 through 1996. They found that for soils initially low in STP, annual applications of 40 kg ha-1 (about 22 mg kg-1) of fertilizer P resulted in higher yields during a three-year period than a single preplant application of 120 kg ha-1 (about 67 mg kg-1) of P.
Soon (1997) used combinations of fertilizer applications prior to forage seeding (preplant) and annual fertilizer treatments of a brome - red clover mix in a fine-textured Gray Luvisol near Beaverlodge. The effect of an annual application of 30 kg ha-1 of P (about 17 mg kg-1) per year resulted in higher yields and higher P uptake than a single preplant application of 90 kg ha-1 (about 50 mg kg-1) of P alone. They concluded that a single preplant application of 45 kg ha-1 (about 25 mg kg-1) of P followed by an annual application of up to 30 kg ha-1of P was the most efficient for crop herbage yield.
The mobility of P is relatively low in the soil, and plant roots take up nutrients primarily from within 2 mm of the root surface (Nye and Tinker 1977). Therefore, banding and seed placement, rather than broadcasting, have been successful methods for improving annual crop response to fertilizer (Malhi et al. 1993; Havlin et al. 1999). In Saskatchewan, Wagar et al. (1986) found the most effective treatment was where an initial broadcast application was used to elevate the STP to optimum levels, followed by small annual applications applied with the seed. For annual crops, regardless of placement methods, it is important to maintain optimum STP levels for best yields (Fixen and Halvorson 1992).
On forage crops, however, Soon (1997) and Simons et al. (1995) reported no beneficial effect of banding vs broadcast of P fertilizer on herbage yield. Banding at depth has been shown to cause serious damage to alfalfa crops (Leyshon 1982), thus, broadcast applications are commonly used to maintain P nutrition once forage crops have been established.
Residual P effects
Total P in surface soils of North America varies from 0.005 to 0.15% (Havlin et al. 1999). Although some prairie soils are high in total P, most prairie soils are low in plant-available P (Havlin et al. 1999). Introduction of P in commercial fertilizer or manure on an annual basis initiates the process of P accumulation in the soil. Since crops do not utilize all the fertilizer P or manure P in the year applied, residual amounts are left in the soil. Subsequent crops, again using only a portion of the annually applied P, use the fraction of the residual P that becomes available that crop year. Long-term annual application of fertilizer P has resulted in an accumulation of inorganic P (Pi) and labile P forms (McKenzie et al. 1992a, b). A decrease in organic P (Po) forms was observed by Tran and N’dayegamiye (1995). Long-term annual manure applications maintained Po levels, but increased total P (Pt) and labile P levels (Tran and N’dayegamiye 1995).
Agricultural soils must have a pool of residual P that is larger than the P uptake requirements for any single crop, in order to ensure adequate crop nutrition. To ensure optimum yields, managers tend to apply P fertilizer at rates in excess of crop demand, causing soil P levels to increase from very low to medium and high during this century (Sibbesen and Sharpley 1997).
Economic analysis to compare optimum STP levels under long-term land tenure to STP levels under short-term land tenure have been presented by Fixen and Halvorson (1991, 1992) and Kastens et al. (2000). Economic advantage could be gained if optimum STP levels increased as the period of land tenure increases. For example, optimum STP levels for land tenure of 20 years should be at least double the optimum STP level for two years. Similarly, in areas where yield potential is high, long-term optimum STP levels should be higher than long-term optimum levels in areas where yield potential is low, such as more arid areas (Fixen and Halvorson 1992). For long-term crop planning, creating a sufficient pool of residual P in the soil was considered part of good economic management, and the benefit can increase if levels are higher in more productive areas.
Starter P fertilizer
In soils with high P levels, withholding P application altogether may not produce satisfactory crop response. Withers et al. (1994) found cereal yields began to decline after three years of no P applications on high P soils. Use of a small amount of commercial fertilizer P as a starter on high STP soils has resulted in crop yield increases in several studies (Griffith 1992; Sibbesen and Sharpley 1997; Havlin et al. 1999; Roberts and Johnston 2001). Griffith (1992) proposed the following possible reasons for crop response to starter P applications that have been observed in soils with high P levels:
- higher crop yields than were obtained in the original calibration studies,
- increased use of minimum tillage,
- increased use of equipment and practices that result in increased soil compaction,
- earlier planting dates into cooler soils, and
- inadequate attention to proper soil pH levels for optimum nutrient efficiency.
Soil conditions appear to be an important factor. Tisdale et al. (1985) found that starter P fertilizer helped early crop growth under cool, wet soil conditions. Griffith (1992) showed forage and grain yields increased with starter P under highly acid soil conditions. Griffith (1992) also found that corn response to starter P in South Dakota was greater in crops following fallow than for recropping.
The use of starter P fertilizer can result in a buildup of soil P levels for several years. Figure 2.1 shows a trend of wheat yield increase with the use of starter P in a fallow - wheat - wheat rotation at Swift Current, Saskatchewan, and a corresponding increase in STP levels even at these low application rates (Roberts et al. 1999).
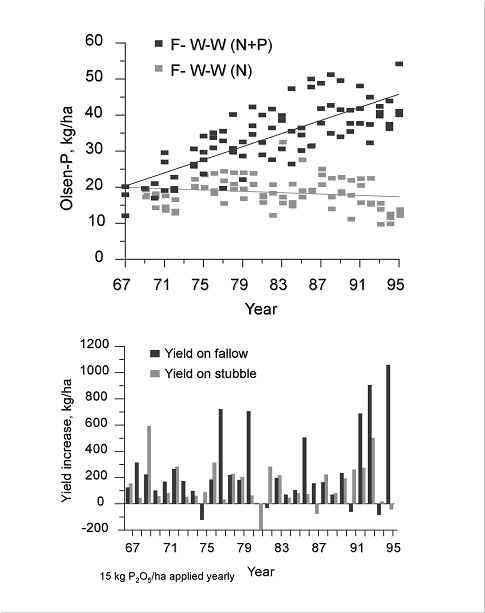
Wheat response to starter P fertilizer with increasing soil test P levels in a fallow -wheat - wheat rotation at Swift Current, Saskatchewan (Roberts et al. 1999).
Relationship between plant-available P and water quality risk
Optimum crop production requires additional input of soil P, and a small buildup of soil P is desirable. Since applications of starter fertilizer can even increase soil P levels with time, care must be taken to ensure that levels do not become high enough to be an unacceptable risk to water quality. Farm managers will be expected to take greater responsibility for maintaining soil P levels for good crop production without unacceptable risk to water quality. However, do farmers have applicable means for assessing risk on their farms?
Ekert et al. (2000) presented criteria which farmers can use to assess and quantify environmental risk. The criteria are based on using the production potential of the land most efficiently, but at the same time, keeping the impacts on soil, water, biota and air within tolerable limits. These criteria include establishing scientifically-acceptable tolerability ranges for risk, development of convincing and feasible actions and goals, and are relatively simple, reproducible and economically acceptable. The concept is holistic and points out that P should not be looked at in isolation of the other fertility factors.
The potential for soil P to impact water quality has been extensively reviewed by Howard et al. (1999). Methods to assess soil P risk, including the Soil P Index, the single limit approach, use of percent saturation, and nutrient management planning were all discussed and evaluated as potential tools for managing P to minimize the risk of degrading water quality. However, finding a practical means to measure soil P and relate its risk to water quality has yet to be agreed upon in the literature.
Because STP is widely used in crop management, a considerable knowledge base exists in understanding how the chemical extraction processes relate to P in different soils. Commercial labs are also set up to perform routine STP analysis at a relatively low cost. As we begin to address environmental issues, will STP be a suitable tool for identifying risk? If producers are to monitor their soils for environmental risk, it would be practical to have environmental soil P levels determined by STP methods, since producers would require only one set of soil samples and one type of analysis. Versions of the Soil P Index use STP as the measurement for soil P in assessing the vulnerability of a site to contribute P to the water system (Sharpley et al. 1999; Hilborn and Stone 2000).
While it may be practical and convenient to use STP as a test for water quality risk, care must be taken to ensure other factors affecting the potential for P transport in surface runoff are considered. First of all, P transport sensitivity requires a different approach to sample collection than that required for soil fertility analysis. For most agricultural soils, samples collected to a depth of 20 to 40 mm would accurately define the effective depth for interaction between surface soil and runoff for rainfall intensities (Coale 1998). Second, some STP tests, such as the Mehlich-3 test which uses strong acids, remove more P forms than just the readily available P fraction (Self-Davis et al. 1998). These tests would, therefore, tend to overestimate the dissolved P (Pd) fraction in runoff. Because of the wide variety of STP tests, care must be taken to ensure any regional environmental guidelines or standards provide correlation for several soil tests that may be used, or have been used, in the region. The third consideration is that environmental risk is not strictly related to dissolved P. While dissolved P is an important water quality parameter, there is a considerable amount of particulate P in runoff that would be undetected (Daniel et al. 1998). This fraction can potentially become available for aquatic plant growth.
Sharpley et al. (1996) suggested that for an environmental test, distilled water or a dilute salt solution (e.g. 0.01M CaCl2) would be better suited to assess the Pd fraction. They stated that such methods would need further evaluation before they could be recommended as a standard procedure. |
|