| | Soil pH is a measure of acidity or alkalinity in the soil. It is measured on a scale from 1 to 14. The higher the number, the more alkaline the soil; the lower the number, the more acidic. A pH of 7 is considered neutral (neither acidic or alkaline). Most crops in Alberta grow best in soil where pH values range from 6.0 to 7.5. Soil pH can affect crop yields along with the physical, chemical and biological properties of soils.
Soil pH influences the availability of plant nutrients (Figure 2.5). Crop growth, quality and/or yield may be affected if any nutrient is lacking in the soil or is not adequately balanced with other nutrients.
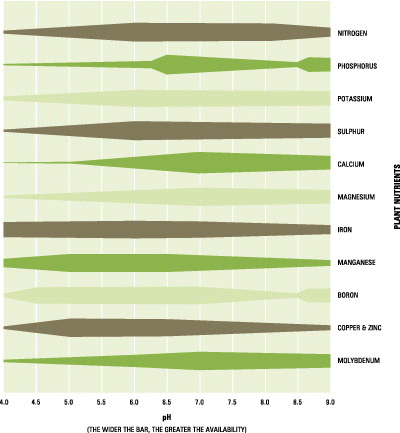
Figure 2.5. Nutrient availability as affected by pH
Adapted from: Agriculture and Agri-Food Canada and Ontario Ministry of Agriculture and Food. 1994.
Best Management Practices: Nutrient Management. Agriculture and Agri-Food Canada and Ontario Ministry of Agriculture and Food. p. 26.
Soil pH can also adversely affect the growth, survival and diversity of soil microorganisms, like nitrogen-fixing bacteria associated with legumes. Some plant species are more acid-tolerant than others (Table 2.3).
Table 2.3. Crop tolerance to acid soils
| Non-tolerant Crops: Alfalfa, sweet clover | Tolerate pH 5.5 to 6.0 |
| Moderately Tolerant Crops: Barley, wheat, rapeseed, alsike, red clover, trefoil | Tolerate pH 5.0 to 5.5 |
| Tolerant Crops: Brome, timothy, creeping red fescue, flax, oats | Tolerate pH 4.5 to 5.0 |
Adapted from: Alberta Agriculture and Rural Development.
Acid soils are most commonly found in central Alberta and in the Peace River region. However, pockets of acid soils can be found in neutral pH areas. The opposite can also occur. Not all soils become acidic. Alberta has naturally high pH soils in the southern dry prairie regions where alkaline (calcareous) subsoils are near the surface.
Factors affecting soil pH:
- Fertilizer applications (particularly nitrogen-based fertilizers and manure) can increase acidity over the long term.
- Plants and soil nutrients are in a complex relationship with soil acidity, increasing pH in some cases and decreasing it in others.
- Peat soils and forest soils are naturally acidic.
- Acid deposition by rain can increase acidity over the long term.
Back to Chapter 2 - Environmental Considerations |
|