| | Introduction | Aster yellows | Sclerotinia stem and root rot | Seedling damping-off | Fusarium root and crown rot | Botrytis blight | Alternaria leaf spot
Introduction
Crop production trends on the Canadian Prairies during the past decade have moved toward diversification and the increased production of exotic herb, spice, essential oil and medicinal plants.
Alberta and Saskatchewan are considered to be among the most desirable areas in the world to cultivate aromatic and medicinal herbs. Factors such as the pristine environment, the availability of high quality agricultural land and the rigorous climate all serve to increase the amount and quality of active ingredients produced in many medicinal herbs.
Echinacea
Echinacea (Echinacea spp.) has recently become one of the most common and highly valued medicinal plants on the Canadian Prairies (Figure 1). The cultivated acreage of echinacea is increasing steadily because of the high value and strong demand for this crop worldwide. There are several commercial plantings throughout Alberta and Saskatchewan.
Echinacea is primarily harvested for its roots, three to four years after planting. Root products are used to strengthen the human immune system and have gained popularity in the health and pharmaceutical food markets.
Three species of echinacea are currently grown in Canada. Narrow-leaved purple coneflower (Echinacea angustifolia DC.) is the most popular because it contains more concentrated active ingredients than the other two species. Purple coneflower (E. purpurea (L.) Moench.) is more popularly grown as an ornamental flower. Pale purple coneflower (E. pallida Nutt.) is not as widely grown. Millions of echinacea plants have been grown under greenhouse and field conditions in the Prairie provinces.

Figure 1. Echinacea is becoming popular
Diseases
Along with new crops can come diseases that have rarely, if ever, been seen on the Prairies. Several new diseases have been found in echinacea plantings in the past few years, but many growers are not yet familiar with their symptoms. These diseases, if not properly controlled, can cause serious losses in echinacea crops.
Early detection could help prevent the spread of these diseases and reduce the damage to standing crops. This factsheet will help growers become familiar with disease symptoms and recommendations for their control.
Based on survey results conducted in Alberta in 1997 and 1998, six major diseases of echinacea were identified:
- aster yellows
- sclerotinia stem rot
- damping-off
- fusarium crown and root rot
- botrytis blight
- alternaria leaf spot
Since many producers of medicinal herbs prefer to grow their crops under organic production systems, the use of biological and cultural pest control measures should be explored along with traditional pesticides.
Aster Yellows
Aster yellows, caused by the aster yellows phytoplasma, was first seen on E. purpurea and E. angustifolia at Brooks in 1994 and 1995. All three species of Echinacea are susceptible to aster yellows. However, E. angustifolia has shown a lower incidence of this disease than E. purpurea in field plots.
The bright yellowing of leaves caused by phytoplasma infection is especially apparent in the early spring when young shoots emerge from the ground (Figure 2).

Figure 2. Phytoplasma infection in young plant of E. angustifolia (right).
Note: yellowing of leaves.
As the disease progresses, symptoms of leaf reddening, plant stunting and proliferation of axillary shoots appear (Figure 3).

Figure 3. Phytoplasma infection causes proliferation of axillary shoots.
Infected plants (left) have a bunchy, or "witch's broom" appearance. Healthy plant is on the right.
In mid-summer, when echinacea typically reaches its flowering stage, floral malformations involving virescence (greening) and phyllody (conversion of floral parts to leaves) are prominent (Figures 4 to 6). Small, secondary, sterile florets issue from the original flower heads.
 |  |
Figure 4. Phytoplasma infection in E. purpurea. Note greening
and distortion in disk and ray florets. | Figure 5. Close-up of an infected flower of E. purpurea with symptoms of virescence (greening) and proliferation of disk florets |

Figure 6. Pytoplasma infection causes distortion and virescence (greening)
of ray and disk florets (left). Normal flower appears on the right.
Diseased plants are subject to winter kill and infections caused by soil-borne pathogens. Symptoms become noticeable by early June and usually occur on two- or three-year-old plants.
Since flower parts are changed into vegetative leaves and sterile florets in the infected plants, no seeds are produced. Therefore, the disease has a tremendous impact on echinacea seed production.
Transmission electron and fluorescent microscopic techniques can be used to look for phytoplasma particles in the phloem tissues.
A large number of phytoplasma bodies are usually present in the sieve tube cells of affected leaves and phylloid flower pedicels (Figure 7). Most of the phytoplasma bodies are spherical to ovoid or irregular in shape, surrounded by single membranes and lack cell walls.
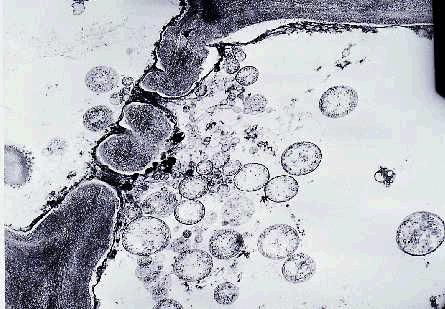
Figure 7. Electron micrograph of phytoplasma bodies (circular to
oval objects) accumulated on one side of a sieve plater of a phloem cell.
Control of aster yellows
The pathogen is transmitted between plants primarily by leafhoppers and by the dividing of roots/crowns of infected plants. Control measures can be taken as follows:
- control and mow down perennial weeds that may harbour phytoplasmas
- apply low-toxicity insecticides such as malathion or pyrethrin onto surrounding weeds to control leafhoppers
- grow echinacea plants with repellent crops such as yarrow, garlic and mint
- select healthy plants for vegetative propagation when dividing crowns and roots
- pull out and destroy plants that show typical symptoms of phytoplasma infection
Sclerotinia Stem and Root Rot
The fungus Sclerotinia sclerotiorum can infect stems and form dark brown to black lesions at and above the soil level. Dead leaves often have bleached petioles. Infected stems and petioles may disintegrate, leaving only fibrous tissues intact. Roots become rotted and black.
This pathogen generates large amounts of white mycelia under moist soil conditions, and black, oblong to irregular-shaped sclerotia (Figure 8) form on the root surface and crown areas after plant death. When infection occurs in the upper stems and shoots, it originates from aerial ascospores and causes stem blotches and dieback (Figures 9 to 11). Diseased plants are easily pulled from the soil.
The pathogen needs moist conditions to establish and intensify the disease. The disease can become very destructive when such conditions exist.
 |  |
| Figure 8a. Wilting of a young shoot of E. purpurea indicating infection with Sclerotinia sclerotiorum. | Figure 8b. Scletorinia blight causes a frayed appearance where the stem breaks. Irregularly-shaped dark bodies (sclerotia) are sometimes found inside or outside the stem and on the crown after the disease has killed the plant |

Figure 9. Sclerotinia blight can cause rapid death in young
echinacea plants when it attacks crowns. Leaves become
brown, dry and brittle.
 |  |
Figure 10a. Sclerotinia blight causes rapid wilting of
adult plants accompanied by drooping of flower heads. | Figure 10b. Advanced symptoms of sclerotinia blight causes the whole plant to collapse. |
 |  |
Figure 11a. Floral infection and dieback of upper stem
of E. angustifolia caused by aerial spores of Sclerotinia sclerotiorum. | Figure 11b. Foliar and stem infection of E. augustifolia caused by aerial spores of Sclerotinia sclerotiorum. |
Control measures
- Crop rotation - Sclerotinia has a very broad host range and can infect many different species of crops. It often requires more than a three year rotation with cereals, grasses or corn crops to reduce the pathogen population in the soil. Therefore, echinacea should not follow canola, sunflower, beans or other susceptible crops.
- Resistant cultivars - E. purpurea is usually more resistant to the pathogen than E. angustifolia.
- Chemical control - No fungicides are currently registered in Canada for controlling this disease.
Seedling Damping-off
This disease is caused by various fungi, e.g. Pythium spp., Rhizoctonia solani, Fusarium spp. and Alternaria spp. Long periods of stratification may predispose seeds to pre-emergence damping-off (Figure 12).

Figure 12. White Pythium mycelium appears on affected seeds after four weeks
stratification at 4°C. Infection spreads from infected seeds to healthy seedlings.
The pathogens occur in contaminated growing media and cause significant losses under cool, damp conditions, which often prevail in greenhouses during the spring (Figures 13 and 14). Diseased plants first show blackening of the root tips, followed by browning of the root and stem, shrinking of the cotyledons and twisting of young leaves (Figure 15). Finally, seedlings wilt and collapse onto adjacent plants.
Under conditions favourable for disease development, seedlings may suddenly collapse due to tissue disintegration at the roots and stem. The disease spreads through contact with infested media, roots or plant materials.
 |  |
| Figure 13. Pythium root rot. Echinacea seedlings showing withered leaves. | Figure 14. Damping-off caused by Rhizoctonia can spread quickly throughunsterilized soil. One-month old seedlings infected with Rhizoctonia solani. Cotyledons and first leaf have lost their turgidity and started to wilt. |

Figure 15. Rhizoctonia root rot. Lower stem appears discolored.
Affected stems may have a smaller diameter than healthy sections, giving the stem a pinched appearance. Cotyledons may senesce prematurely.
Control measures
- Cultural practices - Use pathogen-free growing media and do not overwater seedlings.
- Chemical control - No seed treatments are currently registered for use on echinacea in Canada.
Fusarium Root and Crown Rot
Fusarium spp. (root and crown rot) are the most commonly encountered fungi in infected crowns and roots (Figure 16). This disease usually occurs during the propagation of echinacea by division of crowns and roots. The fungi invade the wounded tissues in the crown or root areas when divided plantings are grown in damp media.
 |  |
| Figure 16a. Crown and root rot of E. pallida caused by Fusarium spp. Discolored lesions on the crown. | Figure 16b. Roots of E. angustifolia affected by crown rot caused by Fusarium spp. Roots have become truncated and several have disintegrated. |
Fusarium oxysporum has been isolated from discoloured vascular bundles of roots and crowns, while F. solani has been found in the cortex. Other species of Fusarium, such as Fusarium venaceum and F. equiseti, have also been isolated from diseased roots.
Control measures
- Avoid injuring roots during cultivation.
- Do not overwater the growing media, especially during the first two weeks after propagation by division of the crown and roots.
- Use healthy plants for propagation.
Botrytis Blight
This disease is caused by the fungus Botrytis cinerea. Under wet conditions, infection often occurs on leaves, especially on the margins or tips and causes water-soaked lesions of various sizes (Figure 17).

Figure 17. Botrytis blight causes brown lesions on leaves of E. angustifolia.
Once the pathogen has invaded the midrib or veins, it can advance rapidly to the petiole and stem, which results in the collapse of the whole leaf. Lesions expand rapidly. The pathogen produces profuse conidia and mycelia on the surface of dead and dying leaves, stems and blossoms (Figure 18). This results in a moldy gray appearance and rapid disease spread.
 |  |
Figure 18a. Infection by botrytis blight causes collapse
of the periole. | Figure 18b. Botrytis blight lesions move up the petioles and into the leaves. Longitudinal lesions appear on the stems. Spores of Botrytis are unicellular, smooth-walled and roughly oval in shape. |
Under dry conditions, the disease develops slowly and forms small to large, brown or black lesions or even becomes quiescent. Large lesions often split leaves or form holes in the center. Round, spherical or irregular microsclerotia may be produced on dead or dying plant parts.
Although B. cinerea does not usually kill coneflower plants, it often heavily infects disc flowers and young shoots. Therefore, botrytis blight could play a significant role in the productivity of this crop under cool, wet growing conditions in the greenhouse and field.
Control measures
- Practice good sanitation by removing infected plant debris at the end of each growing season.
- Grow plants at a proper spacing between and within the rows. Since echinacea plants need three years to reach the harvest stage, proper spacing should be considered at planting to improve air circulation among plants.
- Avoid water condensation in greenhouses. Use proper ventilation systems and water plants before sunset.
- No fungicides are currently registered in Canada for the control of this disease.
Alternaria Leaf Spot
Alternaria spp. infects leaves causing small dark brown- to black-coloured spots (Figure 19), which can grow together and form large lesions under moist conditions. The inner tissue of a large spot may fall out, leaving a hole in the leaf. Alternaria may also infect young shoots and small flowering heads, resulting in die-back symptoms (Figure 20).
Alternaria can infect the seed and become a seed-borne pathogen. Occasionally, the pathogen infects the root, crown and leaves of young seedlings, causing root rot and leaf spots.
Control measures
- Sterilize seeds with a 1% bleach solution for 3 to 5 minutes and rinse them with sterile water before stratification or seeding.
 |  |
Figure 19a. Alternaria causes dark lesions
that tend to follow midrib in seedling leaves. | Figure 19b. Alternaria leaf spots on E. angustifolia. Lesions appear oblong, light-colored in the centre and are surrounded by dark, necrotic tissue. |
 |  |
Figure 20a. Alternaria leaf spot on E. angustifolia.
Lesions appear oblong, light-colored in the centre and surrounded by dark, necrotic tissue. Tissue in the centre
may fall out to create holes in the leaves. | Figure 20b. Alternaria lesions cause collapse of the stem. |
Prepared by:
| Kan-Fa Chang | Ron Howard |
| Field Crop Development Centre | Crop Diversification Centre South |
| Lacombe, Alberta | Brooks, Alberta |
 |  |
| Sheau-Fang Hwang | Stan Blade |
| Crop Diversification Centre North | Alberta Agricultural Research Institute |
| Edmonton, Alberta | Edmonton, Alberta |
Financial assistance to print this factsheet was provided by the Alberta Research Council and the Alberta Agriculture Special Crops Product Team.
Literature cited
- Chang, K.F., R.J. Howard, and S.F. Hwang. 1997. First report of Sclerotinia sclerotiorum on coneflower. Plant Dis. 81:1093.
- Chang, K.F., R.J. Howard, R.G. Gaudiel, and S.F. Hwang. 1997. First report of Botrytis blight, caused by Botrytis cinerea, on coneflowers.
- Plant Dis. 81:1461.
- Chang, K.F., R.J. Howard, S.F. Hwang, R.G. Gaudiel, S.F. Blade. 1998. Diseases of Echinacea in Alberta in 1997. Can. Pl. Dis. Surv. 78:92-94.
- Hwang, S.F., K.F. Chang, and R.J. Howard. 1996. Yellows diseases of echinacea, monarda and caraway. Agdex 630-1, Agri-fax, Alberta Agriculture, Food and Rural Development. 4pp.
- Hwang, S.F., K.F. Chang, R.J. Howard, A.H. Khadhair, R.G. Gaudiel, and C. Hiruki. 1997. First report of a yellows phytoplasma disease in purple coneflower (Echinacea spp.) in Canada. J. Plant Dis. Prot. 104:182-192.
- Peichowski, K., Rizvi, S., and Reese, R.N. 1997. First report of Fusarium oxysporum on purple coneflower. Plant Dis. 81:227.
Source: Agdex 630-2. April 1999. |
|