| | H2S in well water | H2S in dugout water | H2S in water heaters | Water treatment options for removing hydrogen sulphide
Hydrogen sulphide (H2S) is a dissolved gas that gives water a characteristic “rotten egg” taste and odour. It corrodes piping, creates odours in the house and turns water black. Homemakers will notice that it can change sterling silver to black almost instantly. H2S can cause odour problems at a concentration level as low as 0.05 mg/L in well water.
H2S in Well Water
H2S often occurs naturally in well water, or it can be caused by the presence of sulphate-reducing bacteria in a well or water system. Since bacteria are the most common cause, treatment to control them should be tried first. Shock chlorination is the standard treatment for control of sulphate reducing and iron bacteria in a well (see Agdex 716 (D12) Shock Chlorination and Control of Iron Bacteria).
These bacteria also cause “biofouling” problems in water wells. Biofouling problems are related to the slimy bacterial deposits that build up in water wells. These slime deposits plug the intake areas of water wells, which reduces well capacity, as well as reducing water quality. Badly biofouled wells need to be professionally cleaned by a well driller. Simple shock chlorination by the well owner may not yield satisfactory results.
H2S in Dugout Water
H2S may also be present in dugout water, particularly during the winter and early spring. The gas is a by-product of decomposing weeds and algae and is caused by a lack of dissolved oxygen in the water. A good dugout maintenance program combined with dugout aeration will prevent this problem from occurring (see Agdex 716 (B36) Aeration of Dugouts and Ponds with Compressed Air or Agdex 716(B01) Quality Farm Dugouts).
H2S in Water Heaters
Sometimes, H2S may only be present in the household hot water. This condition is caused by a biochemical reaction between sulphates in the water, sulphate-reducing bacteria and a magnesium rod in the hot water heater or organic matter in the water.
If the odour problem in the water heater is caused by heat-loving sulphate-reducing bacteria, disinfect the water heater with chlorine bleach or hydrogen peroxide.
Sometimes, the reaction with the magnesium rod is the cause of the odour problem. The purpose of the magnesium rod is to prevent corrosion of the water heater. Removing the magnesium rod will often prevent the odour problem but will void the warranty and lead to the possible earlier deterioration of the tank. If corrosion is a concern, the magnesium rod can be replaced with a zinc or aluminum rod to reduce the odour problem.
The first thing to try is disinfecting the tank with chlorine bleach or hydrogen peroxide, before using any of the more drastic measures.
Water Treatment Options for Removing Hydrogen Sulphide
Water treatment systems that are effective at removing H2S commonly involve three basic steps:
- changing the form of dissolved H2S in water into a solid using a chemical process
- storing in a tank or length of piping to allow sufficient time for the above change to happen
- filtering to collect any particles formed by the process
Chlorination and filtration
One method to remove H2S is to install a chlorine feeder and a filter (Figure 1). The H2S is oxidized by the chlorine, and any insoluble sulphide particles that form are removed by a filter. Approximately 2 mg/L chlorine must be added to remove 1 mg/L H2S.
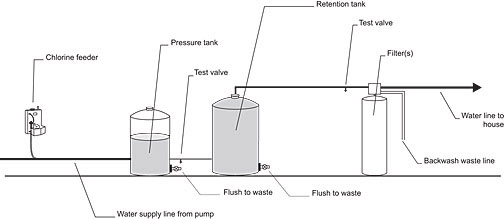
Figure 1. Chlorination - filtration system
The most common system is to install a chlorine feeder and a full-size activated-carbon filter as shown in Figure 1. The chlorine oxidizes the hydrogen sulphide and the activated-carbon filter removes the insoluble sulphide particles. This filter also removes any residual chlorine left after the oxidization of the hydrogen sulphide. This system is most appropriate in situations where there is only H2S present and no significant amount of iron.
If the water also contains a significant amount of iron it is best to have a large sediment filter in front of the carbon filter. The iron should be filtered out before the water enters the carbon filter, which will extend the service life of the activated carbon.
It is important to have enough water flow capacity to backwash the filters. A typical 10 in. (25 cm) diameter filter will require at least 5 gallons per minute (23 litres/ min.) to properly backwash.
The chlorinator is wired to the pressure switch so that the chlorinator is activated when the water pump switches on. The retention tank is installed to ensure sufficient mixing and contact time to complete the oxidation process. The tank should be large enough to retain the water for at least 5 minutes at peak filter capacity (as a minimum, a 42-gallon retention tank is recommended). If iron is in the water, the retention time should be increased to about 20 minutes. A valve should be provided at the bottom of the retention tank to drain any sediment.
Chlorine test valves should be installed just after the pressure tank and just before the activated carbon filter. These valves are needed to help check the chlorine level in the treatment system and to make adjustments to the chlorine feeder.
Manganese greensand iron filter
A properly maintained manganese greensand filter will effectively remove low levels of H2S, typically less than 2 mg/L. This type of filter oxidizes the H2S and filters out the resulting sulphide particles. It is very important that these filters have an adequate supply of water for backwash and are adequately regenerated with potassium permanganate.
H2S requires three times the oxidizing power that iron does, so the greensand filter must be regenerated more often than it would for an equal amount of iron. Manganese stripping, from the manganese greensand, can also occur. This stripped manganese can cause black staining. A chlorine feed system is usually the better choice.
Aeration
Aeration is another option and is accomplished by spraying water into a ventilated storage tank. The H2S gas is separated from the water as it is sprayed and drawn off as a gas by a ventilation system. Aeration will remove most of the H2S, but chlorination may still be necessary. Some sulphide odours will remain due to the high pH of most Alberta waters. The lower the pH, the better this system will work. A second pressure system is required to pump the water from the storage tank into the distribution system.
Although not intended for the job, some aeration-type (chemical-free) iron filters have been used to successfully remove small amounts of H2S. There must be a much higher level of iron than H2S in the water for this approach to work successfully.
Alternative systems
Other oxidizing agents besides chlorine can be added to the water to oxidize H2S. These systems include hydrogen peroxide, potassium permanganate and ozone. Filtration of iron sulphide and iron oxide by-products will still be required after any of these agents have been added.
More Information
Additional information is available through health inspectors, agricultural water specialists or on the web.
The Rural Water Quality Information Tool on the Alberta Agriculture and Forestry website can help assess water test results and provide links to additional factsheets and web sites regarding water treatment.
Alberta Agriculture and Forestry agricultural water specialists can be contacted through the Alberta Ag-Info Centre at 310-FARM (3276).
Prepared by
Farm Water Supply Branch
Alberta Agriculture and Forestry
For more information, contact
Alberta Ag-Info Centre toll-free at 310-FARM (3276) or visit www.agriculture.alberta.ca
Source: Agdex 716 (D14). Revised December 2008. |
|