| | Lady beetles | Green lacewings | Big-eyed bugs | Blister beetles | Parasitic wasps | Parasitic flies | Insect pathogens
This factsheet describes some of the pathogens (diseases), insect predators and parasites attacking field crop insect pests. Natural enemies are those organisms that help control population levels of pest insects.
Predatory insects, such as the lady beetles, eat many prey insects during their development. Lady beetles are just one example of the many different insects that prey, in both larval and adult stages, on other insects. Blister beetles, on the other hand, are predacious only as larvae; the adults are foliage feeders.
Insect parasites include ichneumon wasps and tachinid flies. These insects lay their eggs on, in or near the host insect. The parasite larva develops inside (or sometimes on the outside of) the host insect. The larva feeds on the tissues of only one host individual and usually kills it just before its own larval development is complete. The adults of insect parasites are free living and do not kill insects for food.
Insect pathogens are disease-causing organisms such as viruses, fungi, bacteria and protozoa. These infect the host insect and cause varying degrees of harm, depending on the host and pathogen species, resistance to the disease, environmental conditions and other factors.
Farmers wanting to make better use of the natural enemies of insects in their fields need to distinguish friend from foe. They also need to reduce the number of insecticide applications made to their crops, in order to conserve insect natural enemies. This can be done by integrating various agricultural practices, which also give a measure of control, by choosing sprays that are less hazardous to beneficial insects and by timing the application of sprays to give the best control of pests.
Lady Beetles
The familiar lady beetles (ladybug or ladybird ΠFigure 1) and their larvae are important predators of aphids. They will also eat other plant-sucking insect pests, such as mealybugs, scale insects and spider mites.

Figure 1. Ladybug beetle (top left), and pupa (right) and larva (bottom left). Larva is about to change to pupa. (Alberta Enviromental Centre photo.)
The adults (4-8 mm) are recognized by their nearly round shape and bright colors. Often yellow, red or orange, the beetles usually have a distinctive pattern of black dots on their wing covers.
The larvae are about one centimeter long and look like tiny fat alligators. They are often black or bluish and spotted or banded with bright colors. When the larva is full grown, it attaches itself by its "tail" to a leaf, stem or other surface. The larval skin splits down the back to reveal the pupa, which remains attached to that spot for a few days while the adult is developing within.
The first dramatic success using an imported insect to control a pest insect was achieved in 1888 using a lady beetle called the vedalia beetle. Within two years of bringing the beetles into California, the citrus industry was saved from certain destruction by the cottony-cushion scale (a small sap sucking insect). Today, certain lady beetle species are reared in large numbers in insectaries for sale as "biocontrol agents."

Figure 2. Ladybug larva eating an aphid. (Agriculture and Agri-Food Canada (AAFC), Lethbridge photo.)
The convergent lady beetle, Hippodamia convergens, is a very common and widespread species. It is red with black spots and its name refers to the converging light markings on the thorax. Each larva eats about 25 aphids per day; the adults average 56 aphids per day.
Some species of lady beetles are known to migrate to the foothills and mountains during the fall. There, they assemble in great numbers in sheltered locations to spend the winter. Other species overwinter near forage fields and other places where hosts are abundant. In spring, adults disperse before laying eggs.
Green Lacewings
The green lacewing adult is a delicate looking insect, bright green except for its golden or copper-colored eyes. Its four wings are all similar in shape and size, are net-veined and are folded tent-like over the back when not in use (Figure 3). Green lacewings often give off an unpleasant odor when handled. Both the adults (about 15 mm in length) and larvae (sometimes called aphidlions) are voracious aphid predators.
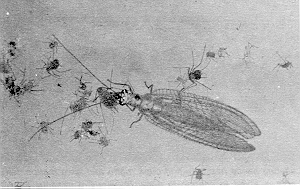
Figure 3. Lacewing adult. (AAFC, Lethbridge photo.)
Eggs are laid on foliage, generally in the vicinity of an aphid colony. Each egg is suspended on top of a thin stalk. Larvae are spiny and alligator-like with long, sickle-shaped jaws to grasp prey and suck their body fluids. Larvae of some species carry shrivelled bodies of prey and bits of trash stuck to spines on their backs. Pupation occurs in small, pea-shaped silken cocoons.

Figure 4. Lacewing larva eating an aphid. (AAFC, Lethbridge photo.)
Green lacewings are easily cultured and have been sold as biological control agents for a number of years, chiefly from insectaries in California.
Big-eyed Bugs
Nymphs and adults of big-eyed bugs are easily identified: their eyes bulge out beyond the edge of the thorax and the tips of the antennae are slightly enlarged. Big-eyed bugs are about 3-5 mm in length and are grey or buff in color. They are somewhat smaller than Lygus bugs, which are green or brown (Figure 5).
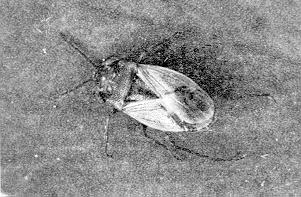
Figure 5. A big-eyed bug, Geocoris bullatus. (AAFC, Lethbridge photo.)
Big-eyed bugs are general predators. They feed on many small insects, such as first instar caterpillars, lygus nymphs and mites, but will concentrate their attack on the numerous species, often aphids or mites. Insect eggs and smaller nymphs are preferred prey. When prey is scarce, big-eyed bugs will feed on nectar from the host plant and to some extent on plant tissues. These predators can then survive until prey become more numerous.
Eggs of big-eyed bugs are laid on leaves, often in a prey colony. Development from egg to adult takes two to three weeks in summer. A complete generation takes from 17 days to a month or more. Either the adult or egg stage overwinters, depending on the species.
Blister Beetles
Blister beetle adults are variously colored, from an iridescent magenta to completely black. They have soft, flexible wing covers and can be distinguished by their shape. The thorax is narrower than either the head or the wing covers, and, in general, the body is long and narrow (Figure 6).
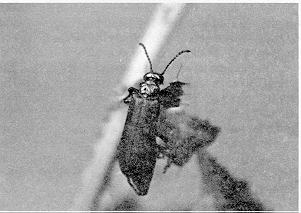
Figure 6. Nuttall's blister beetle, Lytta nuttalli. (Alberta Agriculture, Food and Rural Development (AAFRD) photo.)
Blister beetles are so called because the bodies of some common species contain cantharadin, a substance that causes blisters when applied to the skin. The spanish fly is a common European blister beetle. Bodies of these insects are powdered to make a preparation used medicinally as a skin irritant, diuretic and aphrodisiac.
Blister beetles are abundant during and following years when grasshoppers are abundant because the larvae of many blister beetle species eat grasshopper eggs.
Female beetles construct burrows a couple of centimetres under the soil surface. Masses of eggs are laid in a chamber, and the entrance to the chamber is closed with soil. After a short incubation period, the eggs hatch.
Blister beetle larvae have an unusual development, and they change greatly in appearance as they mature. The first instar larvae are slender, long-legged and seek out egg pods in the soil. As they mature, larvae become grub shaped and less mobile.
Adults are foliage feeders and have caused occasional harm to a variety of crops including potatoes, sugarbeets, cabbage, canola, fababeans and turnips in Alberta. Their damage to plants is made worse by their gregarious nature. Adults swarm and their feeding may be concentrated in small areas, sometimes along field margins or near grasshopper egg beds. Usually the swarms move daily, and control is not normally recommended.
Parasitic Wasps
The ichneumon and braconid wasps are two closely related families of parasitic wasps. All ichneumons (over 3,100 North American species - one of the largest in the insect world) and braconids (over 1,700 North American species) have parasitic larval stages and free-living adults.
Ichneumon wasps range in size from 4 to 38 mm while braconids tend to be smaller (2-15 mm). The adults are colored in black and browns, and many ichneumons are reddish or yellowish as well and brightly patterned. The ovipositor of ichneumon females is thread-like, and for those species that lay their eggs in the tunnels of wood-boring grubs, may be several times the length of the body. Other than a color spot on the leading edge of each front wing, the wings are clear and prominently veined.
Many of our common insect pests have ichneumon or braconid wasp parasites. The bertha armyworm parasite, Banchus flavescens, an ichneumon, attacks early-stage bertha larvae, grabbing them with her legs and inserting her ovipositor through the caterpillar's skin to lay an egg. The wasp larva develops slowly at first, to allow the host to grow to maturity.
Once the parasitized bertha tunnels into the soil to pupate, the parasite rapidly completes its development, kills the host and chews an exit hole through the cadaver. The mature parasite larva then spins a cocoon within the earthen cell provided by the bertha. The parasite larva overwinters and pupates in the spring. This parasite is a major controlling factor in bertha armyworm populations.

Figure 7. A red-backed cutworm killed by ichneumon parasite larvae. The larvae emerged from the cutworm and spun cocoons (seen below the cutworm cadaver) from which the wasps emerged. (AAFRD photo.)
The alfalfa weevil ichneumon, Bathyplectes curculionis, was imported from Italy into the U.S. in 1911 after the discovery of alfalfa weevils in 1904. The parasite feeds internally in the same manner as the bertha armyworm ichneumons. It was found in Alberta alfalfa fields shortly after the weevils were first found (1954). The parasite is credited as being an important factor in the control of alfalfa weevil in Alberta.

Figure 8. A pea aphid mummy and its emerged broconid. (Aphidius) parasite. (AAFC, Lethbridge photo.)
Parasitic Flies
Tachinid flies are a very common group (over 1,300 North American species) and are easily recognized.
Many of the tachinids are large (5-15 mm in length), dark-colored, exceedingly bristly and may appear bee-like. The larvae of all species are parasites of the larvae of butterflies and moths, beetles, sawflies and other insects. Most tachinids lay their eggs directly on the body of the host.
The tachinid larva hatches, burrows into the host and feeds internally. When fully developed, it leaves the host and pupates nearby. Other tachinids lay their eggs on foliage where they are eaten by leaf-eating hosts. The eggs hatch in the host's gut, and the parasite larvae burrow into the body through the gut wall.
The first instars of some parasite species lie in wait and grab onto hosts as they pass by. There are native species of tachinids that attack such important pests as the cutworms, armyworms and tent caterpillars. A tachinid native to the Orient and Europe was imported into Canada in 1923 for European corn borer control.
Flesh flies appear similar to tachinids but have grey and black stripes along the thorax. The larvae of these flies have more varied habits than the tachinids. Many are scavengers, feeding on dead animal matter; some are parasites of insects.
Grasshoppers are parasitized by certain flesh fly species whose females can attack hopping or flying grasshoppers and lay a newly hatched larva on the grasshopper. The larva burrows into the hopper and feeds internally. When the fly larva is mature, it pushes its way out of the hopper's body through the neck, thereby decapitating its host, or through the soft skin between the wings and pronotum.
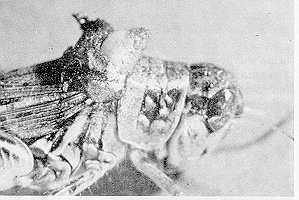
Figure 9. Mature flesh fly maggot emerging from its grasshopper host. (AAFC, Lethbridge photo.)
Insect Pathogens
Insects are infected by many disease-causing organisms (pathogens) including viruses, fungi, bacteria and protozoa. Some pathogens are quite common and frequently cause widespread infection in insect populations. Other pathogens are rare.
The effect on the host insect may be severe, and the pathogen may inflict high mortality on the population. On the other hand, some pathogens may produce only mild, long term effects. Pathogens vary in their specificity to the host. Some viruses will infect only one species or several closely related species. The bacteria, Bacillus thuringiensis, on the other hand, infects the larvae of numerous species of butterflies and moths.
Pathogens have already found a place in our arsenal of weapons for insect control. Bacillus thuringiensis (or BT) has been produced in commercial formulations since 1958. It provides control of many butterfly and moth larvae such as cabbage looper, imported cabbageworm, alfalfa looper and diamondback moth. When BT is applied with insecticides, lower rates of insecticides can be used, and more insect enemies of the host remain to provide continuing control.
Nuclear polyhedrosis virus (NPV) has also been effectively used against the larvae of butterflies and moths. The virus invades many tissues in the insect including the blood cells, fat body and linings of the breathing tubes. As infection progresses, the larva becomes sluggish, and its skin discolors and may appear oily. Before dying, the larva will often climb to the highest point available. In death, the corpse hangs from the food plant - a bag of liquified tissues that ruptures, contaminating the food plant with millions of virus particles.
Alfalfa looper is susceptible to virus infection, and outbreaks of this insect are brought under control by the virus. Unfortunately, the virus does not kill the loopers until they are nearly mature and have caused their damage. Solutions to this commonly observed drawback with natural control may be to artificially inoculate the population early or to increase the susceptibility of early instar larvae to disease.
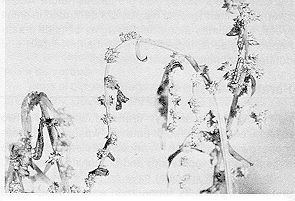
Figure 10. Clover cutworms killed by a virus infection. (AAFRD photo.)
Various types of fungi are parasites of insects and can either kill their insect hosts outright or reduce their ability to reproduce. In addition, invasion of an insect by a fungus will leave the insect weakened and susceptible to infection by other disease causing organisms, such as bacteria, or to poisoning by insecticides. These fungi are valuable natural enemies in the fight against pests.
Fungi do not attack insects indiscriminately but are specific to a select group of insects. The fungus Entomophaga grylli (it has no common name) is known to attack certain pest grasshoppers. Another Entomophaga species attacks pea aphids. Strongwellsea castrans is a species that infects canola root flies and, as its name suggests, leaves the adult flies unable to reproduce normally.
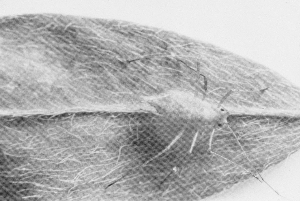 | 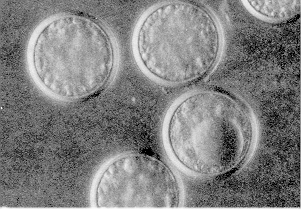 |
| Figure 11. Pea aphid killed by a fungus infection. (AAFRD, Lethbridge photo.) | Figure 12. Entomophaga fungus resting spores. Twenty-five laid end to end would measure 1 mm. (AAFC, Lethbridge photo.) |
Naturally occurring disease-causing protozoa are numerous, widespread and are known to devastate insect populations. As with other insect pathogens, these protozoa affect only certain species; that is, they have narrow host ranges. It is hoped that recurring and costly outbreaks of some agricultural pests may be controlled using these protozoa.
Grasshoppers can become diseased by a protozoan called Nosema locustae, which occurs in trace levels over much of the grasshoppers' North American range. During grasshopper outbreaks, the pathogens also multiply and can reduce host populations to low levels. Infected grasshoppers produce fewer eggs per pod and fewer pods because the disease organisms compete with the grasshopper for the food reserves in its body.
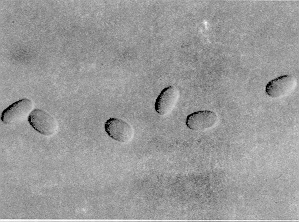
Figure 13. Spores of Nosema locustae, a protozoan pathogen of grasshoppers. Two hundred spores laid end to end would measure 1mm. , (AAFC, Lethbridge photo.)
Various programs have studied the potential of applying large doses of Nosema to grasshopper populations. Very large doses act like an insecticide and kill grasshoppers outright but leave other insects unharmed. Lower doses reduce the grasshoppers' ability to reproduce and feed. The effects of Nosema have been studied in combination with other natural enemies and with insecticides. The results show that Nosema will be a viable agent for control when integrated with other compatible grasshopper control agents.
Source: Agdex 620-3. |
|