| | Conditions for iron bacteria growth | Shock chlorination treatment | Effectiveness of shock chlorination | Shock chlorination procedure for drilled wells | Modified procedure for large-diameter wells
Iron bacteria are a common nuisance in water wells, but are not considered a health hazard. They use dissolved iron in the water as an energy source and leave slimy deposits of red iron hydrate as a by-product.
This slime will coat the inside of the well casing, water piping and equipment, creating problems such as reduced well yield, restricted water flow and red staining of plumbing fixtures and laundry. However, all iron- staining problems are not necessarily caused by iron bacteria. The iron naturally present in the water can also cause significant problems.
Conditions for Iron Bacteria Growth
Iron bacteria thrive in water that contains 0.5 to 4 mg/L of dissolved oxygen and as little as 0.01 mg/L dissolved iron.
They prefer a temperature range of 5 to 15°C. Water wells will almost always produce these conditions.
Iron bacteria also create an environment that encourages the growth of sulfate-reducing bacteria in the well. Some of these sulfate-reducing bacteria can produce hydrogen sulfide as a by-product, resulting in a “rotten egg” or sulfur odour in the water. Others produce small amounts of sulfuric acid, which can corrode well casing and pumping equipment.
The easiest way to check a well and water system for iron bacteria is to examine the inside surface of the toilet flush tank. If a greasy slime or growth is present, then iron bacteria are probably present.
Shock Chlorination Treatment
Shock chlorination is used to treat iron and sulfate- reducing bacteria in a water system. To be effective, shock chlorination must disinfect:
- the entire well depth
- the formation around the bottom of the well
- the pressure system
- some water treatment equipment
- the distribution system
To accomplish this result, a large volume of superchlorinated water is poured down the well to displace all the water in the well and some of the water in the formation around the well. Figure 1 shows a typical water system.
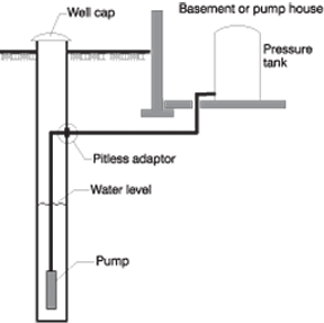
Figure 1. Typical water system
Effectiveness of Shock Chlorination
With shock chlorination, the entire system (from the water-bearing formation, through the well bore and the distribution system) is exposed to water that has a concentration of chlorine strong enough to kill iron and sulfate-reducing bacteria. Bacteria collect in the pore spaces of the formation and on the casing or screened surface of the well. To be effective, you must use enough chlorine to disinfect the entire cased section of the well and adjacent water-bearing formation.
Liquid chlorine solutions can deteriorate by 20 to 25 per cent per month in storage. Always use “fresh,” newly purchased household bleach or 12 per cent sodium hypochlorite. Due to the short shelf life of these products, it is not a good idea purchase more than you are immediately going to use.
The described procedure does not completely eliminate iron bacteria from the water system, but will hold it in check. To control the iron bacteria, you must repeat the procedure each spring and fall as a regular maintenance procedure. If your well has never been shock chlorinated or has not been done for some time, it may require two or three treatments before you notice a significant improvement.
Shock Chlorination Procedure for Drilled Wells
Caution: If your well is slow yielding or tends to pump any silt or sand, you must be very careful using the following procedure. Over-pumping a well that pumps sand may damage the well. To avoid this problem, siphon the solution down the well very slowly and pump the well out very slowly.
Step 1. Store sufficient water to meet farm and family needs for 8 to 48 hours.
Step 2. Pump the recommended amount of water (see Table 1) into a clean water storage container. A clean galvanized stock tank or pick-up truck box lined with a 4 mil thick plastic sheet is suitable. The recommended amount of water is the amount required to provide twice the volume of the well casing. To calculate the depth of the water in the casing, refer to the water well driller’s report. Subtract the “static water level” from the “total depth of the well.” If this information is not available, use a water well depth sounder to find the static water level. Always disinfect the well sounder before and after use.
Example: Drilling records indicate the casing is 250 ft. deep, and the static water level is 150 ft. The length of casing with water is 100 ft. (250 -150). If your casing is 6 inches in diameter, you need to pump 2.4 gal. of water for every foot of water in the casing into your storage container. Since you have 100 ft. of water in the casing, you will pump 2.4 gal./ft. x 100 ft. = 240 gal. of water into the clean water storage container.
Using Table 1, calculate how much water you need to pump into clean storage.
| Table 1. Amount of chlorine required to obtain a chlorine concentration of 200 ppm 4,5 |
| Casing diameter | Volume of water needed | 5.25% domestic chlorine bleach1 | 12% industrial sodium hypochlorite | *70% high test hypochlorite |
Water needed per 1 ft.
(30cm) of water in
the casing | Litres needed per 1 ft. (30cm) of water | Litres needed per 1 ft. (30cm) of water | Dry weight2 per 1 ft. (30cm) of water |
| inches | millimetres | gallons | litres | litres | litres | grams |
| 4 | (100) | 1.1 | 5 | 0.019 | 0.008 | 1.44 |
| 6 | (150) | 2.4 | 10.9 | 0.042 | 0.018 | 3.12 |
| 8 | (200) | 4.2 | 19.1 | 0.072 | 0.032 | 5.46 |
| 24 | (600)3 | extra 200 gal. | extra 1,000 L | 0.34 | 0.148 | 25.4 |
| 36 | (900)3 | extra 200 gal. | extra 1,000 L | 0.76 | 0.34 | 57.2 |
1 Domestic chlorine bleach should not have additives or perfumes. Use “fresh,” newly purchased bleach only.
2 Since a dry chemical is being used, it should be mixed with water to form a chlorine solultion before placing it in the well.
3 See modified procedure for large diameter wells.
4 To reduce the chlorine concentrations to 50 PPM, divide the above chlorine amounts by 4.
5 More chlorine is not better. Excess chlorine may be detrimental.
Step 3. Mix the recommended amount of chlorine with the water. Equivalent strengths of chlorine are shown in Table 1. This works out to be 200 ppm chlorine solution.
Example: If your casing is 6 inches in diameter and you are using 12 per cent industrial sodium hypochlorite, you will require 0.018 L per foot of water in the casing. If you have 100 ft. of water in the casing, you will use 0.018 L x 100 ft. = 1.8 L of 12 per cent chlorine.
Step 4. Siphon this solution into the well. (See Figure 2.)
Step 5. Open each outlet (including dishwashers, washing machines, etc.) in the water distribution system until the water coming out has a chlorine-like odour. Note: you may want to bypass treatment equipment to prevent damage. Check with your water treatment supplier.
Step 6. Leave the chlorine solution in the well and distribution system for 8 to 48 hours. The longer the contact time, the better the results.
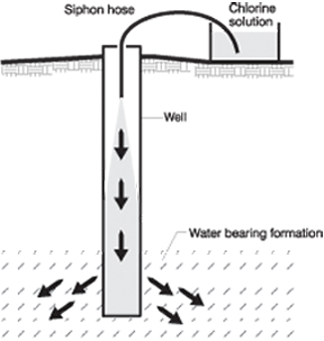
Figure 2. Syphoning water down a well.
Step 7. Open an outside tap and allow the water to run until the chlorine odour has disappeared.
Step 8. Flush the chlorine solution from the hot water heater and household distribution system. The small amount of chlorine in the distribution system will not harm the septic tank.
Step 9. Backwash and regenerate any water treatment equipment.
Use the worksheet at the end of this factsheet to determine how much water and chlorine you need to shock chlorinate your well.
Modified Procedure for Large-Diameter Wells
Due to the large volume of water in many bored wells, the above procedure can be impractical. A more practical way to shock chlorinate a bored well is to mix the recommended amount of chlorine right into the well. An extra 200 gallons of chlorinated water is then used to force some of the chlorine solution into the formation around the well.
Follow these steps to shock chlorinate a large-diameter bored well.
Step 1. Pump 200 gal. (1,000 L) of water into a clean storage tank at the well head.
Step 2. Mix 4 L of 5.25 per cent domestic chlorine bleach (or 1.6 L of 12 per cent bleach or 0.3 kg of 70 per cent calcium hypochlorite) into the 200 gal. of stored water. This mixture will be used later in Step 5.
Step 3. Using Table 1, calculate the amount of chlorine you require per foot of water in the casing and add directly into the well. (Note that the 70 per cent hypochlorite powder should be mixed with water to form a solution before syphoning it into the well.)
Step 4. Circulate chlorine added to the water in the well by hooking a garden hose up to an outside faucet and placing the other end back down the well. This method circulates the chlorinated water through the pressure system and back down the well. Continue this procedure for at least 15 minutes.
Step 5. Syphon the 200 gal. bleach and water solution prepared in Steps 1 and 2 into the well.
Step 6. Complete the procedure as described in Steps 5 to 9 for drilled wells.
Other Sources of Information
All of the above are available from:
Alberta Agriculture and Forestry
Publications Office
7000 - 113 Street
Edmonton, Alberta T6H 5T6
Toll free: 1-800-292-5697
Worksheet for calculating water and chlorine requirements (200 ppm) for shock chlorination
Complete the following table using your own figures to determine how much water and chlorine you need to shock chlorinate your well.
| Worksheet |
| Casing diameter | Volume of water needed | 5.25% domestic1 chlorine bleach | 12% industrial sodium hypochlorite | 70%2 high test hypochlorite |
in. | mm | Imperial gallons needed per 1 ft. (30cm) of water in the casing | Litres needed per 1 ft. (30cm) of water | Litres needed per 1 ft. (30cm) of water | Dry weight2 per 1 ft. (30cm) of water |
4 | (100) | ____ ft. x 1.1 gal. = ____ | ____ ft. x 0.019 L = ____ | ____ ft. x 0.008 L = ____ | ____ ft. x 1.44 g = ____ |
6 | (150) | ____ ft. x 2.4 gal. = ____ | ____ ft. x 0.042 L = ____ | ____ ft. x 0.018 L = ____ | ____ ft. x 3.12 g = ____ |
8 | (200) | ____ ft. x 4.2 gal. = ____ | ____ ft. x 0.072 L = ____ | ____ ft. x 0.032 L = ____ | ____ ft. x 5.46 g = ____ |
24 | (600)3 | extra 200 gal. | ____ ft. x 0.340 L = ____ | ____ ft. x 0.148 L = ____ | ____ ft. x 25.40 g = ____ |
36 | (900)3 | extra 200 gal. | ____ ft. x 0.760 L = ____ | ____ ft. x 0.34 L = ____ | ____ ft. x 57.20 g = ____ |
1 Domestic chlorine bleach should not have additives or perfumes.
2 Since a dry chemical is being used, it should be mixed with water to form a chlorine solultion before placing it in the well.
3 See modified procedure for large diameter wells.
Prepared by
Alberta Agriculture and Forestry
For more information
Alberta Ag-Info Centre
Call toll-free: 310-FARM (3276)
Website: www.agriculture.alberta.ca
Source: Agdex 716 (D12). Revised February 2007. |
|