| | List of materials needed for active C test | Revised protocol for active C field method | Calculations used to determine standard curve equation
List of Materials Needed for Active C Test
| Aluminum trays to dry soil samples | Metal scoop to level off measuring spoon |
| Large brown glass bottle (>1L) | Timer |
| 3 smaller brown glass bottles | Centrifuge tubes |
| pH meter | Lab tissues |
| Small scoop (5 ml aluminum measuring spoon) | Plastic basin |
| Weigh paper | 2 glass cuvettes |
| CaCl2 (500g container) | 10 % bleach solution |
| 550 nm colorimeter | Labels |
| KMnO4 (500 g container) | Distilled water |
| 0.1M NaOH (1 liter bottle) | Plastic cup |
| Bulb pipettes | Stir stick |
| Funnel | Graduated cylinder |
| Felt marker |  |
.
Revised Protocol for Active C Field Method
Mixing the stock solution:
| 1) | Combine 147.01 grams CaCl2*2H20 into 1 L distilled water in a dark glass bottle. |
| 2) | Add 31.6 grams KMnO4 to mixture described above. |
| 3) | Add small amounts of NaOH with a bulb pipette and after each addition test the solution until a pH of 7.2 is reached (between 5 and 10 drops in 1 L of solution, test after each drop). |
To test pH, we immersed the pH meter in the solution corresponding to the basic or acidic reading needed and then rinsed the meter with distilled water. We placed approximately 15 ml of the stock solution in a plastic cup and tested the pH. If the ideal pH had not been reached we rinsed the pH meter and the cup with distilled water. We then added another drop of the NaOH solution and re-tested until the desired pH was reached.
| 4) | Place a label on the bottle and using a marker, note the contents for safety. |
 |  |
| . |  |
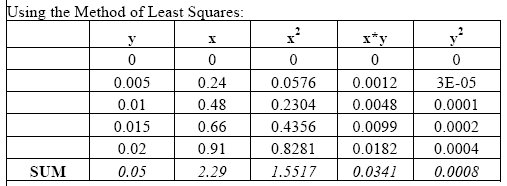
The standard solutions were prepared two times a day, in the morning and afternoon. This procedure differs from that of Weil et al. (2003) in that we added a fourth standard solution, 0.015M, in order to create a more accurate standard curve.
| 1) | Take 1.25 ml (29 drops) of the stock solution and place it in a clean brown bottle. Dilute the solution to the 50 ml mark with distilled water, using the graduated cylinder to measure. Label the bottle 0.005M. |
| 2) | Take 2.5 ml (58 drops) of the stock solution and place in clean brown bottle. Dilute the solution to the 50 ml mark with distilled water, using the graduated cylinder to measure. Label the bottle 0.01M. |
| 3) | Take 3.75 ml (86 drops) of the stock solution and place in clean brown bottle. Dilute the solution to the 50 ml mark with distilled water, using the graduated cylinder to measure. Label the bottle 0.015M. |
| 4) | Take 5 ml (115 drops) of the stock solution and place in a clean brown bottle. Dilute the solution to the 50 ml mark with distilled water, using the graduated cylinder to measure. Label the bottle 0.02M. |
| 5) |
- Fill a clean glass cuvette with distilled water. Wipe the outside of the vial with a tissue. Place vial in the colorimeter (generic 550 nm colorimeter, Hach® Company, Boulder, CO) well and put the cover in place. Press the zero button and after a few seconds the LED should read 0.00. Remove the cuvette and rinse it with distilled water (If the reading is higher after 10 to 20 determinations clean the cuvette with a 10% bleach solution).
- Add 49.5 ml of distilled water to a clean centrifuge tube. Using the disposable bulb pipette specifically for the standard solution of 0.005M KMnO4 place 0.5 ml (12 drops) of the standard into the tube. Place the cap on the tube and shake for a count of 15.
- Pour approximately 15 ml of the diluted standard into a 20 ml glass cuvette, filling the cuvette and emptying it three times with the diluted standard, wipe the outside with a tissue and place in colorimeter well. Put the cover in place and press the read button. Record the absorbance displayed.
- Rinse the cuvette thoroughly with distilled water.
- Repeat steps 5 (a-d) using 0.5 ml of the 0.01M, 0.015M, and 0.02M standard solutions. Record the absorbance for each standard solution.
|
| 6) | Create standard curve using readings. |
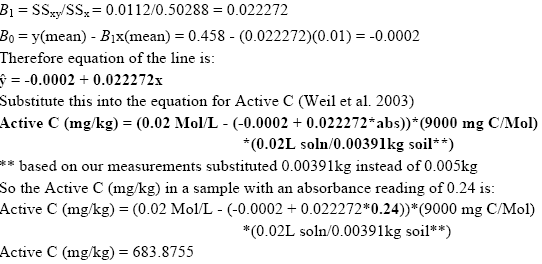
To create the standard curves we first plotted the absorbance reading for each of the standard solutions. Using the method of Least Squares (Mendenhall 1983) we obtained the standard curve equation. We then used these results in the equation provided by Weil et al. (2003) to calculate the active C content of all soil samples (Active C (mg/kg) = (0.02Mol/L - (a+b*absorbance))*(9000mg C/Mol)*(0.02L soln/0.005kg soil). Please note that based on our findings we used 0.00391 kg soil in place of the 0.005 kg used in the above equation by Weil et al. (2003).
To test the soil samples, we followed the procedure described below:
| 1) | Soil samples were laid out to air dry in aluminum tins for a period of 24 hours. After this time each sample was checked to ensure it was dried thoroughly. If any sample was still wet it was left to dry for longer. |
| 2) | Before the tests were begun the temperature of the lab was taken in the morning and afternoon using a thermometer (Checktemp-1 pocket thermometer, Hanna® instruments, Laval, Quebec) and recorded. |
| 3) | Using a pipette place 2.0 ml (46 drops) of the stock solution in a clean 50 ml centrifuge tube. Add 20 ml of distilled water to the tube, using the graduated cylinder to measure the correct amount of water. Cap the tube, and using a sharpie mark the site name on the lid. Swirl the tube for a count of 15 to mix the solution thoroughly. |
| 4) | Add one level scoop of uniformly dry soil to the tube and cap it. |
| 5) | Shake the tube vigorously (~ 100 strokes/min) for 2 minutes, set the timer to ensure that each tube is shaken for a consistent amount of time. |
| 6) | Stand tube in rack and set the timer for 10 minutes to allow the soil to settle. Ensure the rack is not in direct sunlight. |
| 7) | After the ten minute settling time is up add 49.5 ml of distilled water to a clean centrifuge tube labeled with the corresponding site name of the first tube. |
| 8) | Using a clean bulb pipette take 0.5 ml (fill pipette) of the liquid from the upper 1 cm of the soil-KMnO4 solution (first tube) while avoiding floating debris, and place 12 drops (0.5ml) in the tube with distilled water. |
| 9) | Cap the tube and shake it for a count of 15. |
| 10) | Pour approximately 15 ml of the diluted solution from the second tube into a clean glass cuvette swirl and dump contents and repeat two times. Fill cuvette with the diluted solution. |
| 11) | Wipe the outside with a lab tissue and place in the colorimeter well. Put the cover in place and press read. |
| 12) | Record the absorbance for the solution on the datasheet (if all the KMnO4 has reacted, i.e. no detection on the colorimeter, repeat steps 1-5 and 7-13 using only 0.25 g of dry soil). |
In order to speed up the process a maximum of seven tubes were done at once. This was achieved by placing fourteen tubes in the rack and filling seven with 20 ml of distilled water and the other seven with 49.5 ml of distilled water. We used a graduated cylinder to measure out the volumes of distilled water to add to the centrifuge tubes to reduce the variability caused by measuring based on the markings on the tubes.
Forty-six drops (2 ml) of the stock solution were then added to the tubes with 20 ml of water, the lids labeled and then the tubes capped and shaken. A scoop of the corresponding soil sample was placed in the appropriate tube, ensuring that the scoop and the metal leveler were wiped after each sample to avoid cross-contamination. When the 10-minute settling period was up the procedure outlined above was followed for each tube. Two glass cuvettes were used during this process. One was filled with distilled water and was used to zero the colorimeter after every five samples (a reading of this cuvette was also taken after five samples to ensure it read as 0.00 on the colorimeter). The second glass cuvette was used specifically for the soil-KMnO4 solutions. It was not rinsed with distilled water after each sample as each solution was rinsed through the cuvette three times to ensure that no solution from the previous sample remained.
At the end of each day all the bottles containing the standard solutions and the scoop were cleaned with distilled water and set out to dry. The glass cuvettes were rinsed and cleaned with a 10% bleach solution and set out to dry.
Calculations Used to Determine Standard Curve Equation
Example from readings taken in the morning of January 30, 2004
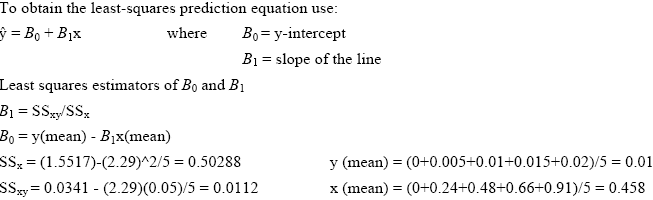
 |
|